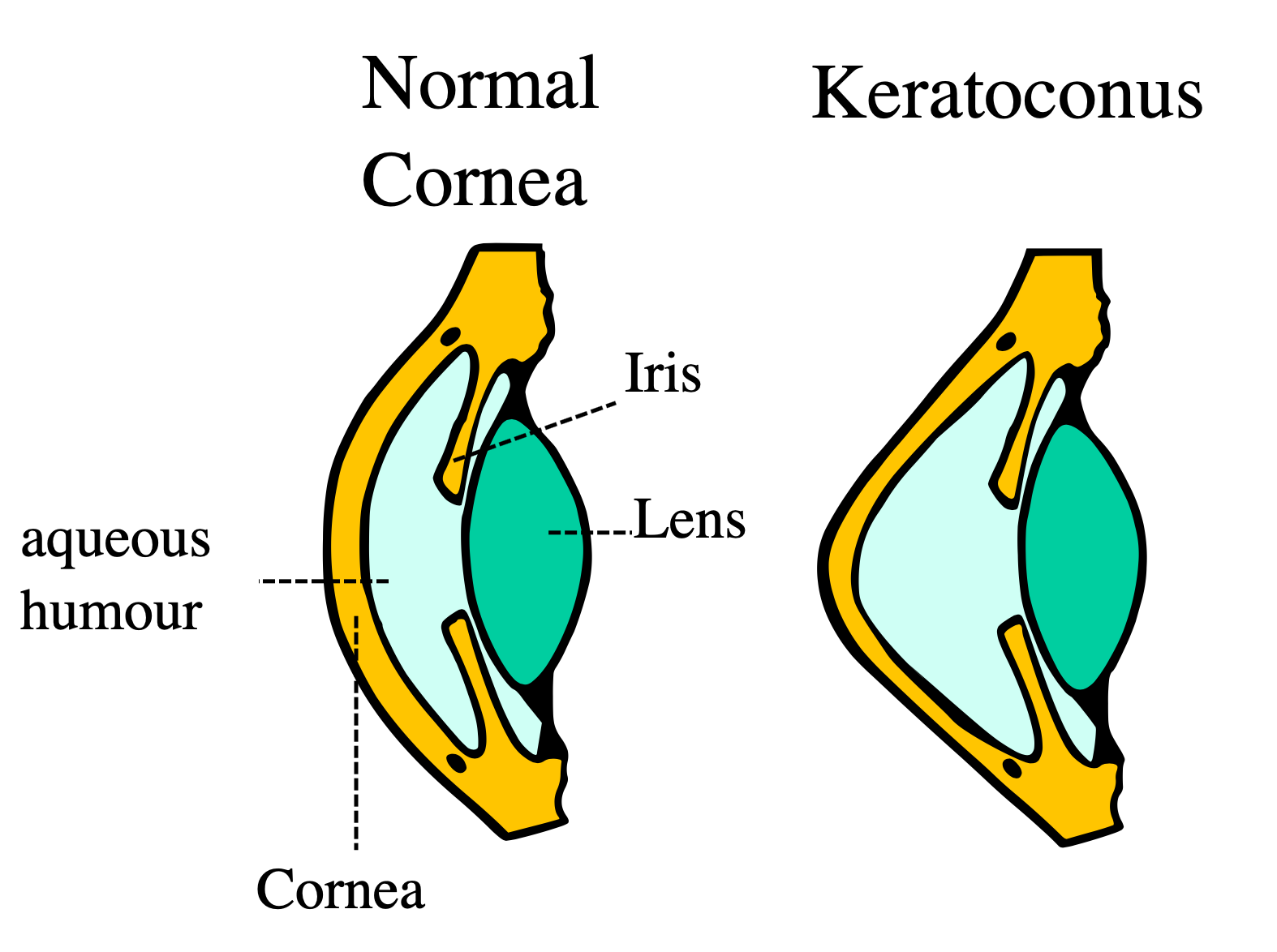
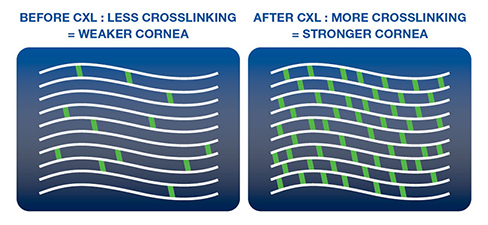
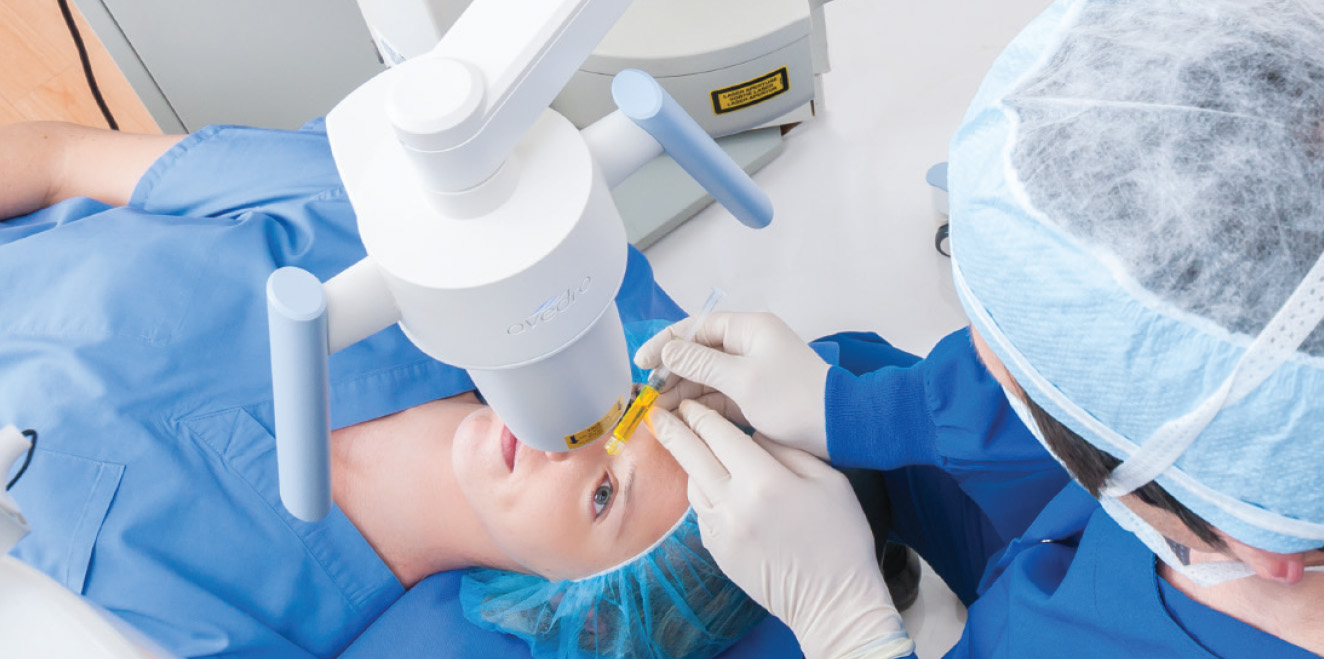
Corneal Cross-linking (KXL) at ClearSight
Here at ClearSight, our goal isn’t to perform a procedure; it’s to deliver life-changing results. When we recommend cross-linking as your treatment option, our service includes everything you need to get you the best possible outcome. For KXL this includes:
• Your initial Corneal Cross-linking (KXL) Procedure
• Basic follow-up exams for two years so that we can assess healing and stability
• A starter kit of prescription eye drops
• A 20% discount on procedures such as PRK or EVO ICL to improve vision (good for 2 years)
If you would like to learn more about cross-linking, or find out if you’re a candidate, please schedule your complimentary eye exam and consultation with us today! Get started by picking your preferred day and time below: